Abstract
Pinus thunbergii is a popular species of conifer cultivated as bonsai. In an effort to produce superior surface roots on P. thunbergii, bonsai enthusiasts have developed a method of making cuttings from young seedlings to replace any taproots with radially distributed lateral roots. These lateral roots are developed to improve the look of the tree when it matures and to help develop taper or flair near the base of the trunk. The focus of the present study is to measure the effect of different treatments on the production of roots developed through the “seedling-cutting” technique.
Cuttings were made from 63 seedlings of P. thunbergii planted 84 days previously. Treatments tested include 1-Naphthaleneacetic acid (Dip ‘n Grow), 1-Naphthaleneacetamide (Rootone) and a low dose fertilizer (Olivia’s Cloning Gel).
Root treatments outperformed controls by a small margin when product instructions were followed. Increased exposure to hormones stimulated increased root production.
Introduction
Bonsai enthusiasts appreciate Japanese black pines, Pinus thunbergii, for the age and character conveyed through their bark as much as for their general shape and appearance. A pine’s surface roots play an important role by demonstrating the tree’s connection to the ground in which it grows.
As black pine bonsai became more popular, producers developed approaches to creating trees with superior surface roots. By making a cutting of a young seedling, a grower can essentially replace the taproot with several lateral roots that are distributed radially. This approach has become known as the “seedling-cutting” technique.
The idea behind the present study is to guide the production of pines created by the seedling-cutting technique. While seedling-cuttings can be developed without the use of hormones, their application can produce significantly more roots than seedling-cuttings made without them.
This study is limited to the comparison of basic approaches to creating seedling-cuttings and does not allow for specific recommendations for producing superior roots. It is intended as preliminary research aimed at guiding future efforts to optimize root production for bonsai in P. thunbergii and Pinus densiflora.
Materials and Methods
Creating the Seedling-Cuttings
P. thunbergii seeds were sown in a bed containing equal parts of lava, pumice and clay pebbles (particle size was between 1mm – 5mm) and covered with a thin layer of sand (particle size 1mm – 2mm) on February 9, 2013. After 84 days the seedlings were uprooted and the taproots were removed with a razor blade leaving approximately 2.5cm of stem. The cut is made with a slicing action to avoid crushing stem tissue. Cuttings were placed in water after the taproots were removed to prevent them from drying out.

Figure 1. P. thunbergii seedlings 84 days after planting in seedbed of lava, pumice and clay pebbles covered with thin layer of sand. The red-colored stems indicate optimal time for making seedling-cuttings.

Figure 2. Seedling-cutting.
Cutting Treatments
Dip ‘n Grow Treatment: Cuttings were placed in a vial with Dip ‘n Grow (1-Naphthaleneacetic acid 0.05% at 10x dilution) for 5 seconds per the manufacturer’s instructions. A second batch remained in the vial for 5 minutes to measure the effect of increased exposure.

Figure 3. Seedling-cutting to be dipped in Dip ‘n Grow.
Rootone Treatment: Cuttings were dipped in Rootone (1-Naphthaleneacetamide 0.2%) and excess powder was flicked off.

Figure 4. Rootone.

Figure 5. After dipping seedling-cutting in Rootone.

Figure 6. After flicking off excess hormone.
Olivia’s Cloning Gel Treatment: Cuttings were dipped in gel (N-P-K: 0.08-0.15-0.09) before planting.

Figure 7. Seedling-cuttings in cloning gel.
Control: Cuttings were planted with no hormone or other treatments.
Cutting at the First Root: A variation of the control, these seedlings were cut below the first root. Taproots were removed leaving a single lateral root.

Figure 8. Removing taproot below first lateral root.
Planting the Seedling-Cuttings
Cuttings were planted in a bed of lava, pumice and clay pebbles similar to the bed used for germination. Instead of sand, channels of small clay pebbles (particle size 1mm – 2mm) were used to provide additional moisture for the cuttings.

Figure 9. Planting medium used to grow seedling-cuttings.
The bed was watered before planting and perforations were made with a 2.5mm wire. Treated cuttings were placed in these perforations and sand was added to hold the cuttings in place. Sections of 1.5mm aluminum wire were used to pin down cutting foliage to prevent the cuttings from moving during watering. Seedling-cuttings were kept outdoors under 30% shade cloth and watered when the planting medium began to dry out.

Figure 10. Aluminum wire secures the cuttings.

Figure 11. After planting, the 63 seedling-cuttings were gently watered.
Results and Discussion
The seedling-cuttings were watered regularly throughout summer, fall and winter. In winter, some seedlings showed signs of yellowing, a sign of stress due to temperature, growing conditions, fungus and/or infestation. Seedling-cuttings were uprooted 295 days after planting when the apical buds began to extend.

Figure 12. Seedling-cuttings 295 days after planting, February 23, 2014.
Mortality was low overall (6.3%) but significantly higher among the control and cut to one root seedling-cuttings (25%) than among the treatment group (3.6%). As mortality could be attributed to bird activity as well as cutting treatment, mortality was not considered as a factor contributing to study results.
The seedling-cuttings were evaluated by counting primary root divisions. Results for the control group and the group cut back to a single lateral root showed no difference, each producing 2-3 lateral roots per seedling-cutting. The cloning gel performed slightly better than the control, whereas the hormone treatments produced better results. The treatment that produced the greatest number of roots was the group that soaked in the Dip ‘n Grow solution for 5 minutes, 60 times the recommended exposure.

Figure 13. Seedling-cutting treated with Dip ‘n Grow – 5 minute treatment.
The reason for taking two approaches to the Dip ‘n Grow treatment is that it offered the best flexibility in terms of managing exposure to the cutting treatment. Holding a stem in powder longer does not allow for greater hormone absorption. Exposure to gel nutrients remains the same regardless of time spent in the gel as it sticks to the stem after it is removed. Stem tissue has some ability to absorb liquid hormone as demonstrated by the increased root production resulting from increased exposure to the liquid Dip ‘n Grow.
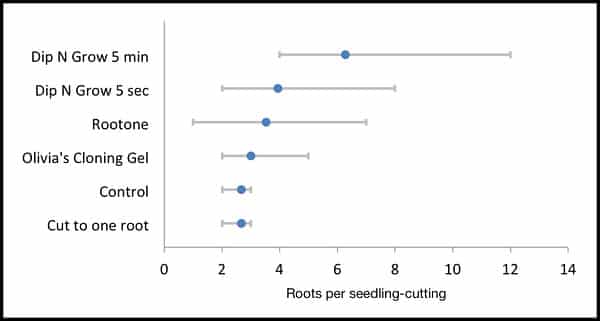
Figure 14. Average number of roots per seedling-cutting. Range indicates minimum and maximum values.
This study suggests that varying the time spent in a liquid hormone solution may provide some flexibility for controlling root production. Producers wanting more surface roots could increase exposure to hormones while producers wanting fewer could limit exposure. Further study is required to determine expectations for root production based on varying levels of hormone exposure.
Directions for Further Study
In addition to suggesting a correlation between hormone exposure and root production, this study shows that a variety of approaches can produce seedling-cuttings acceptable for use as bonsai. Further inquiries could add to these findings by:
- Measuring the effect of increased exposure to hormones to offer guidance relating root production to exposure time. This would allow growers to control root production to fit their needs.
- Measuring the effect on stem length for the cutting. Does leaving longer or shorter stems make an appreciable difference?
- Varying the time at which seedling-cuttings are made.
- Modifying the planting environment to ensure all seedling-cuttings receive equal warmth and moisture. Planting all seedling-cuttings in one containers makes the amount of light received equal but cuttings planted near the sides of the container receive more warmth and dry out more quickly whereas cuttings towards the center of the pot have more moisture available to them.
- Measuring overall root mass and/or plant mass to determine which approach leads to the most vigorous trees.
- Varying the timing and types of fertilizer applied during the growing season.
- Varying the growing medium and/or exposure to sunlight to determine optimal conditions for increasing vigor.
- Determining the optimal number of surface roots for trees of different styles and sizes. This is a broader and more subjective area of inquiry that is tied more closely to the fundamental purpose of creating bonsai as objects for aesthetic appreciation.
Supporting Information
Roots Per Cutting
| Treatment | Low | High | Avg |
| Dip N Grow 5 minutes (n=7) | 4 | 12 | 63 |
| Dip N Grow 5 seconds (n=16) | 2 | 8 | 39 |
| Rootone (n=15) | 1 | 7 | 35 |
| Olivia’s Cloning Gel (n=15) | 2 | 5 | 3 |
| Control (n=3) | 2 | 3 | 27 |
| Cut to one root (n=3) | 2 | 3 | 27 |
Table S1. Low, high and average number of roots per seedling-cutting.

Figure S1. Dip ‘n Grow – 5 minute treatment

Figure S2. Seedling-cutting treated with Dip ‘n Grow for 5 seconds

Figure S3. Control specimen.
Roots Per Cutting
| Treatment | Seedling #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | #11 | #12 | #13 | #14 | #15 | #16 |
| Dip ‘n Grow – 5 seconds (n=16) | 2 | 5 | 5 | 2 | 5 | 3 | 4 | 3 | 8 | 3 | 2 | 3 | 6 | 5 | 4 | 3 |
| Olivia’s Cloning Gel (n=15) | 2 | 3 | 2 | 5 | 3 | 1 | 3 | 3 | 3 | 2 | 4 | 3 | 4 | 2 | 5 | 0 |
| Rootone (n=15) | 4 | 7 | 3 | 4 | 3 | 6 | 3 | 3 | 2 | 3 | 2 | 5 | 1 | 4 | 3 | 0 |
| Control (n=3) | 3 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cut to one root (n=3) | 3 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dip ‘n Grow – 5 minutes (n=7) | 7 | 4 | 6 | 5 | 12 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table S2. Number of primary root divisions for seedling-cuttings observed 295 days after cuttings were made. “x” indicates seedling-cuttings that died or disappeared.

Figure S4. Dip ‘n Grow – 5 minute treatment

Figure S5. Dip ‘n Grow – 5 second treatment (seedling-cuttings 1-8)

Figure S6. Dip ‘n Grow – 5 second treatment (seedling-cuttings 9-16)

Figure S7. Rootone (seedling-cuttings 1-7)

Figure S8. Rootone (seedling-cuttings 8-15)

Figure S9. Olivia’s Cloning Gel (seedling-cuttings 1-7)

Figure S10. Olivia’s Cloning Gel (seedling-cuttings 8-15)

Figure S11. Cut to one root

Figure S12. Control group.
Acknowledgements: The author wishes to thank Boon Manakitivipart and Kathy Shaner for introducing him to the seedling-cutting technique as well as Kindai Bonsai and Bonsai Today for publishing articles on the topic.
Author Contributions: Conceived and designed the experiments: JD. Performed the experiments: JD. Analyzed the data: JD. Wrote the manuscript: JD.
Keywords: Conifers, Hormones, Horticulture, Lateral roots, Plant roots, Seedlings
Citation: Dupuich J (2015) Effect of hormone treatments on P. thunbergii cuttings for the production of surface roots on trees cultivated for bonsai. Bonsai Tonight. https://bonsaitonight.com/2015/07/17/effect-of-hormone-treatments-on-p-thunbergii-cuttings-for-the-production-of-surface-rootage-on-trees-cultivated-for-bonsai/
Funding: This research was funded by the author.
Competing Interests: The author has declared that no competing interests exist.
Copyright: © 2015 Dupuich. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Subscribe to Bonsai Tonight
New Posts Delivered Every Tuesday and Friday
Mac McAtee says
Jonas, Thank you for taking the time to perform this experiment and for writing it up and making it public.
Thomdec says
This is great info. Time for an experiment. I am going to test the powdered hormone on some Coast Live Oak seedlings-cuttings. The timing seems right, we’ll see. This is part of what makes bonsai so much fun!
Thanks for sharing.
Bonsai Scratcher says
A fascinating investigation. Thanks
japanesepots says
Excellent work Jonas. Good protocol, good analysis. Wouldn’t liked to see some more in depth analysis about the radial or non radial nature of the testees….even the better control you picture is a bit bowlegged 😉
Great work. Solid science.
jroskind1 says
Truly a very well constructed and reproducible study.
Bravo
Alex says
This is an incredible article, Jonas.
Mark says
Wouldn’t it be better if each group (according to the chemical used) had been planted in a separate container instead of all of them being planted into the same one? This way there was a possibility of “contamination” through the growing medium.
I’m just asking because this article looks like it wants to have aspirations of botanical merit. The oversight I’m describing considerably reduces it’s value.
Jonas Dupuich says
Hi Mark – very good point, I do think it would have been better if each group was planted in a separate container. I don’t know how long the hormones persist or how they might move through the growing medium or if they did how one might inter/counteract with another. I’d planned a follow-up with better protocols but opted to not make seedling-cuttings with last year’s batch and wanted to share what I’d done so far.
Thank you for the feedback – it’ll help with the next experiment!
Mark says
Not that I think that having only one container had any measurable impact, but the article looks like it could have been published in some botanical publication. Well, maybe backed by a repeated experiment with larger numbers of seeds / seedlings / chemicals / species / time-tables / etc. If that’s the case, it should really be as accurate as possible. Otherwise some highly strung botanist may find a reason to look down on bonsaists. Don’t get me wrong, I’m rooting for bonsaists in this case.
Having said that, in your case I also like the fact that you’re dealing with seeds / seedlings, which is a no-no in a strictly bonsai world. You might find highly strung bonsaists looking down on such behavior.
Jonas Dupuich says
Hi Mark – thanks again for the feedback. I think your comments about the study are apt and I definitely want to stay on botanists’ good side as I’d appreciate all the help from them we can get.
I appreciate your comment about seeds and seedlings too. I get that seedlings aren’t good for learning to wire or style bonsai and that the process is far from easy, but I wouldn’t want to discourage anyone with the interest from pursuing it and am happy to share what I can on the topic 🙂
dirk says
Jonas, i’ve followed your posts for a long time now. You gave me the info i need to have more success with seedlings and seedling-cuttings of JBP. This is the second year of taking seedling cuttings for me. Last year only 2 out of 10 took. This year only 1 of 60 failed (so far). I’m experimenting with growing medium (akadama or pure perlite) and type of container (mesh bottom, polystyreen box, pond basket). The number in my experiment is rather small so not sure i can attribute something. Thanks for the tip for fixating with a wire after taking the cuttings. I’ve seen them turn while watering but eventually they took. Thanks for guiding us. Dirk
Marty says
Jonas – How many seedlings were in each group initially? You commented a bit or mortality rate, but it would be nice to see the details. It also appears to me that the group sizes were not all the same based upon the total number and the size of some of the groups. This is fine, but it would be good to document it.
Jonas Dupuich says
Hi Marty, thanks for the note. In going through this again I noticed that I reported one more seedling than I actually planted which changed the rates a bit (updated above). I also added “x”s to Table S2 to show which seedling-cuttings didn’t make it. I planted 16 each of the Dip ‘n Grow (5 seconds), Rootone and cloning gel, 7 Dip ‘n Grow (5 minutes), 4 cut to one root, and 4 control.
James Frank says
As I was reading your study it occurred to me that considering the inherent variability among subjects within groups, statistical analysis might provide deeper insight to the interpretation of the data. Therefore, after testing the data distributions for normality, I analyzed the data you reported using one-way ANOVA with post hoc adjustment for multiple comparisons with Dunnett’s test. Because the sample sizes for the two control groups were so small and appeared identical, I combined them to improve the statistical power to detect differences. This analysis shows that only the dip n grow 5 min treatment is statistically different from the control and other groups. None of the other groups was significantly different from control. Based on this finding I would propose that only your 5 min dip and grow treatment actually increased the number of primary root divisions more than mere chance alone. If a readers objective is to achieve a larger number of primary root divisions, it appears that only that treatment could be relied upon to do the trick. It remains possible that one or more of the other treatments might also have some affect on this outcome, but the magnitude of the effect would be small and perhaps not useful. In other words, it is possible that increasing the sample sizes might reveal that a significant difference exists between control and the other treatments, but difference between the means of the groups would not likely be larger than what you have reported (eg 1-2 more divisions if any). I’ve included a graph below showing the data in a slightly different way than you presented. I thought you might find this of interest because in contrast to your primary conclusion, this analysis suggests that only one of the treatments actually produced the desired effect.